Open and Closed Systems in Thermodynamics
The study of energy energy transformations and its relation to matter. A perfect isolated system is tough to return by but an insulated drink cooler with a lid is conceptually almost like a real isolated.

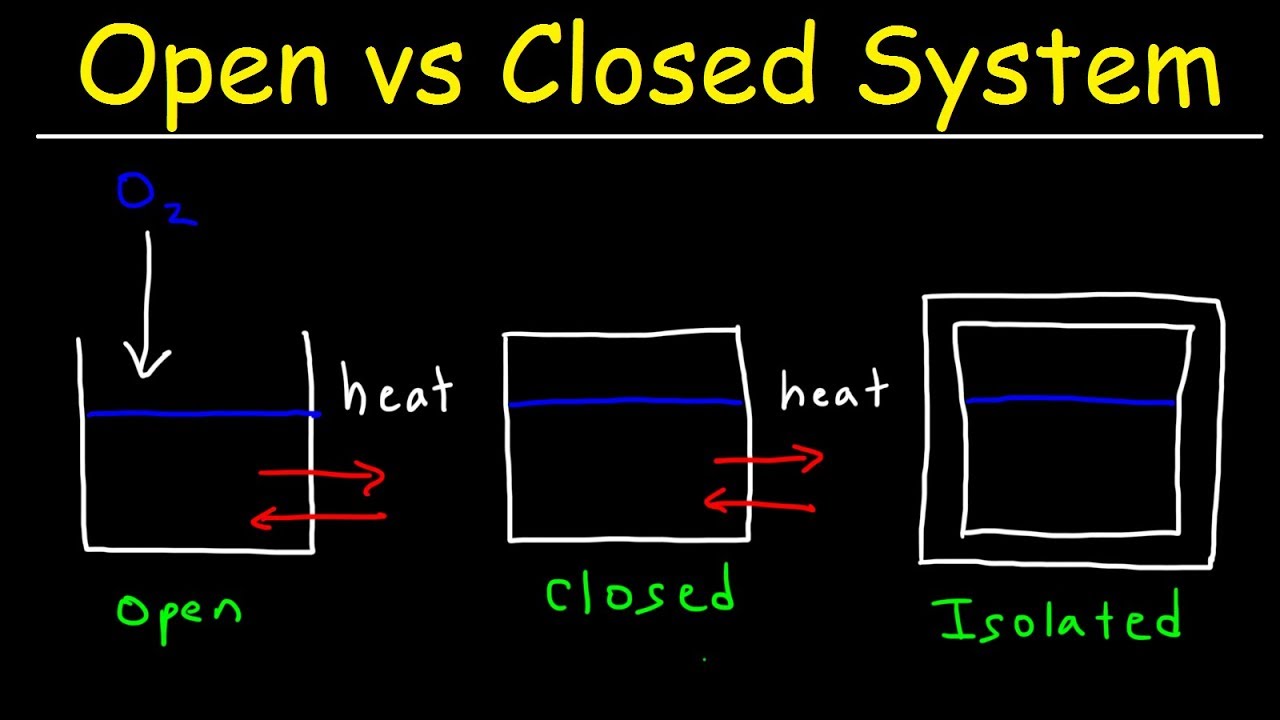
Open Vs Closed Vs Isolated Thermodynamic Systems Thermodynamics Internal Energy Free Energy
The anal-ysis of thermal systems is achieved through the application of the governing conservation equations namely Conservation of Mass Conservation of Energy 1st law of thermodynam-ics the 2nd law of thermodynamics and the property relations.
. Ch 9 - Power Systems. 24 OPEN SYSTEMS ENTHALPY KINETIC AND POTENTIAL ENERGY In closed systems the fluid particles move from one end of the container to another but they do not leave the container. 4F-2 - Efficiency of a Power Cycle.
It is impossible to have a net effect on the velocity and therefore on the kinetic energy of the system. 4F-3 - Coefficient of Performance of a Refrigeration Cycle. The only truly isolated system being the universe itself.
In open systems fluid particles move from. Ch 8 - Thermodynamics of Flow Processes. In thermodynamics the concept of energy is broadened to account for other observed changes and the principle of conservation of energy is extended to include a wide variety of ways in which systems interact with their surroundings.
Isolated systems essentially do not exist. Sub-critical systems where five might be closed heaters with one deaerator. Ch 5 - The First Law of Thermodynamics.
Ch 6 - The Second Law of Thermodynamics. The only ways the energy of a closed system can be changed are through transfer of energy by work or by heat. Ch 7 - Entropy.
The first law of thermodynamics provides the definition of the internal energy of a thermodynamic system and expresses its change for a closed system in terms of work and heat. The second law is concerned with the direction of natural processes. There are three types of systems in thermodynamics.
Lesson A - Conservation. Ch 4 - The First Law of Thermodynamics. 4F-4 - Heat and Work for a Cycle Executed in a Closed System Containing Ammonia.
There are three sorts of systems in thermodynamics. Isolated System - No exchange of energy or mass between an isolated system and its surroundings. Back to Top of this Page.
The universe is a solitary system. A closed system in thermodynamics is one where heat is trapped inside the system as we saw in the vacuum thermos flask example. 4F-1 - Heat and Work for a Cycle Carried Out in a Closed System.
Open closed and isolated. It asserts that a natural process runs only in one sense and is not reversible. It can be linked to the law of conservation of energy.
Closed isolated and open. Ch 5 - The First Law of Thermodynamics. Thermodynamics show that work and efficiency of a steam generator improve with increased pressure.
Closed systems are much simpler to understand than open systems.

Open System Closed System And Isolated System Thermodynamics Physics Thermodynamics Chemistry Education Physics

Different Types Of Thermodynamic Systems Open System Closed System Isolated S Sponsored Paid Affiliate Thermodynamic Thermodynamics System Chemistry

Types Of Thermodynamic Systems Thermodynamics System Electrical Engineering
No comments for "Open and Closed Systems in Thermodynamics"
Post a Comment